Technological knowledge

The wonder of Superoxide Dismutase is its ability to seize and to provide free radical electrons. The so-called Disproportionation Reaction can effectively remove free radicals out of organisms.
And blue-green algae – the very first organism that initiated photosynthesis – brought the Great Oxygenation Event to earth 2.5 billion years ago. It converted the O2 in atmosphere from originally 1% or less to 20%, which opened the prologue of biological evolution. During respiration, living organisms turn partial oxygen (O2) into Reactive Oxygen Species (ROS), and further produces various types of free radicals. In order to resist against the harms caused by free radicals, blue-green algae evolved to generate Superoxide Dismutases (SODs).
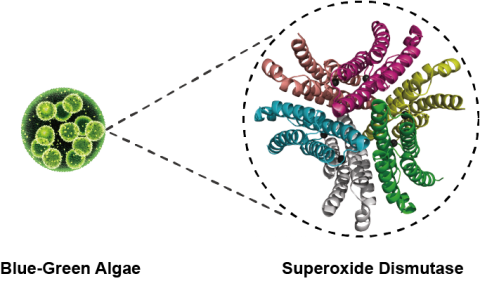
Fig. 1 The Protein Structure of SOD inside Blue-Green Algae
In 1969, Fridovich and McCord discovered that SOD widely exists in various types of animals, plants, and microorganisms. It is a very important antioxidant inside organisms as it prevents cells from damages or mutation caused by ROS.
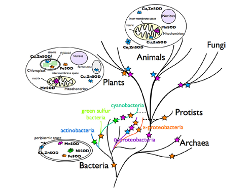
Fig. 2 SOD Species Diagram
When the concentration of superoxide ions inside organisms increases, Superoxide Dismutase will perform disproportionation reaction and convert superoxide ions into O2 and H2O2 (when pH = 7, the reaction speed is approximately 105 M−1s−1).
The SOD-catalyzed superoxide disproportionation reaction can be divided into two steps:
- Oxidation Reaction: M(n+1)-SOD + O2− → Mn+-SOD + O2
- Redox Reaction: Mn+-SOD + O2− + 2H+ → M(n+1)-SOD + H2O2
The “M” can be metal ions such as Cu(n=1), Mn(n=2), Fe(n=2), Ni(n=2), hence SOD includes 4 activity centers such as copper/zinc, manganese, iron, or nickel. During such reactions, the oxidation state of metals performs oxidation reaction and redox reaction within n and n+1, in order to remove ROS effectively.
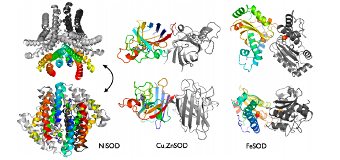
Fig. 3 Comparison of the ribbon structures that characterize the three families of SOD.
Latest
Clean Beauty: An important Trend You Need To Know
Let's take a look at this new trend!